INTRODUCTION
As a direct result of changes in technology and practices in medicine, the frequency of opportunistic infection has increased dramatically in recent years. Oral fungal infections have increased in frequency as a result of the widespread use of immunosuppressive drug therapies. At one time oral fungal infections were a relatively uncommon event, but with advances in health care and an increasingly aging population, oral fungal infections are becoming a more routine clinical finding.
Fungal diseases have long affected plants, animals, and human hosts. As early as the fourth century BC, Hippocrates, the Father of Medicine, described fungal disease affecting humans. Since then, the approach to diagnosis and management of these diseases has become more systematic. To aid in classification, management, and treatment, human fungal infections are now routinely grouped into superficial, subcutaneous, or systemic categories based chiefly on the site of clinical involvement.
Superficial oral fungal diseases
The most commonly occurring oral fungal infection is caused by Candida species with Candida albicans most often encountered. Candida is an obligate organism found in the digestive and vaginal tracts of human hosts. In fact, up to 60% of all healthy ambulatory, non-immunocompromised individuals may harbor this organism in the oropharyngeal region. Candida is a dimorphic fungus that can exist both in a yeast phase (blastospore, blastoconidial) and a hyphal (mycelial) phase. Candida reproduces by multilateral budding and may develop either as a mycelia form, composed of long, branching septae or filaments, or as spherical or ovoid yeast cells. Dimorphism is relevant to pathogenicity of the yeast and to the clinical problems of diagnosis and treatment of fungi. The presence of sufficient quantity of Candida can indicate a pathogenic state and can occur beyond the oropharyngeal region. For instance, Candida species are the fourth most common cause of nosocomial bloodstream infections in the United States.
Candida species are assumed to cause disease by direct tissue invasion, either by inducing a hypersensitive state or by producing potent Candida toxins. Oral Candida infections, commonly referred to as candidiasis or oropharyngeal candidiasis, are one of the most common conditions affecting the oral mucosa. C albicans usually accounts for 70% to 80% of oral isolates; however, non–C albicans species such as C glabrata and C tropicalis account for approximately5%to8%of oral isolates, respectively. Healthy individuals with yeast colonization have, on average, 300 to 500 colony forming units (cfus) of Candida per milliliter of saliva. The quantification of salivary Candida cfus varies with a diurnal and daily variation. The tongue, followed by the palate and buccal mucosa, are the more densely yeast populated areas of the oral cavity. Poor oral hygiene is associated with increased carriage of yeast.
The symptoms associated with oral Candida infection can range from none to a painful burning sensation that may interfere with the ability to swallow (dysphagia) and ability to take in nutrition. Oral Candida infections have traditionally been stratified into four clinical subgroups: erythematous, pseudomembranous, hyperplastic, and angular cheilitis. More recently, a revised classification system that stratifies Candida lesions into two subgroups, group I and group II, has been proposed (Box 1). Group I lesions are localized to the oral cavity and do not involve any other cutaneous or mucosal sites. Clinically, group I lesions consist of three different presentations: pseudomembranous, erythematous, and hyperplastic. There are also group I Candida-associated lesions, such as angular cheilitis, central papillary atrophy, and denture stomatitis, and keratinized lesions of other disorders that are commonly superinfected with Candida. Group II lesions are also known as secondary lesions and may have oral and extraoral sites of infection. Group II lesions are typically associated with rare inherited immune defects.
Classification of group I candidal lesions
Pseudomembranous candidiasis
Pseudomembranous candidiasis, commonly referred to as thrush, was first described by Langenbeck in 1839 as a fungus in the buccal aphthae in a patient with typhus. Clinically, pseudomembranous candidiasis can appear as white or yellow plaques on any mucosal surface (Figs. 1 and 2).
The plaques represent an overgrowth of the yeast on the mucosa leading to epithelial cell desquamation and a concomitant accumulation of bacteria, keratin, and necrotic tissue. These lesions can be either confluent or discrete. The plaques are readily removable leaving a raw underlying surface. Pseudomembranous Candida is most often found in persons with immune system dysregulation, such as infants, elderly persons, those with immune diseases, and individuals taking immunosuppressive medications.


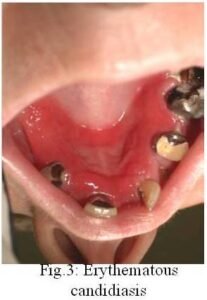
Erythematous candidiasis
Erythematous candidiasis is sometimes referred to as ‘‘atrophic candidiasis.’’ The term atrophic originates from the Greek term for ill-fed, atrophos. Typically this variant of candidiasis appears as erythematous patches on any mucosal surface (Fig. 3). Most often, the affected surfaces are the palate and dorsal tongue, where it can cause depapillation. Erythematous candidiasis often is most associated with the use of broadspectrum antibiotics or corticosteroids. Increased presence of neutrophils is associated with the clinically noted inflammatory reaction.
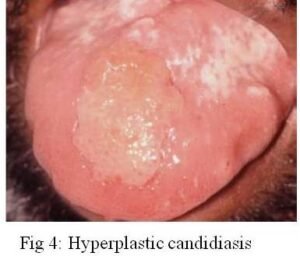
Hyperplastic candidiasis
Hyperplastic candidiasis (chronic hyperplastic candidiasis or candidal leukoplakia) is the third clinical candidal variant. It is typically described as a white plaque presenting on the commissural region of the buccal mucosa, and it cannot be readily wiped off (Figs. 4). Others sites affected in decreasing order include buccal commissures, palate, and tongue. According to several authors, chronic hyperplastic Candida infection is associated with a diagnosis of dysplasia. Samaranayake and MacFarlane suggest that up to 15% of all chronic hyperplastic candidal infections may progress into dysplasia. The role of Candida in epithelial dysplasia is unclear and may in fact be related to a host environmental change as a result of the dysplasia. The possibility that Candida may be associated with epithelial dysplasia requires that patients with chronic, recalcitrant hyperplastic Candida infections be followed closely.
Candida-associated lesions
Denture stomatitis or denture sore mouth is a chronic inflammatory condition of the denture-bearing mucosa caused in part by the erythematous variant of Candida. The porosity of the acrylic allows fungal and bacterial contamination throughout the entire denture. Moreover, the relatively acidic and anaerobic microclimate underneath the denture provides an ideal environment for yeast growth, and the denture shields the mucosa from the saliva and its local immunity-enhancing properties. Thus, the denture or any acrylic-containing dental appliance can serve as a source of inoculation for bacteria and fungi. Disinfection of dentures and dental appliances plays a key role in eradicating this form of candidal infections.
Central papillary atrophy (median rhomboid glossitis) has also been associated with chronic Candida infection. Central papillary atrophy is relatively uncommon, with a prevalence less than 1%. Men are affected three times more often than women. The condition clinically appears as a rhomboid-shaped hypertrophic or atrophic plaque in the mid-dorsal tongue. Clinical infection with Candida at other mucosal sites is not associated with central papillary atrophy.
Angular cheilitis is a mixed bacterial fungal infection that is commonly noted at either or both corners of the mouth. Other agents associated with angular cheilitis include Staphylococci and Streptococci. The typical presentation is erythematous fissuring at the commissures often times accompanied by a pseudomembranous covering. Angular cheilitis may also affect the anterior nostril region. Contributing factors include facial wrinkling along nasolabial folds and reduced occlusal vertical dimension, both of which promote a moist environment conducive to the growth of cheilitis-causing agents. Another, less-cited factor contributing to angular cheilitis is nutritional deficiency. Thiamine, riboflavin, iron, and folic acid have all been implicated in cases of angular cheilitis.
Host factors associated with candidiasis
Oral candidiasis is associated with a decrease in the host defense mechanism. This decrease in the defense mechanism may have many causes and may be local or systemic in nature. One local cause for reduced host defense mechanisms is the use of topical medications. For example, inhaled corticosteroids are now routinely used to suppress airway inflammation in the management of bronchial asthma and have reduced or eliminated the need for systemic corticosteroids. Inhaled corticosteroids are associated with an increased risk for oral candidiasis, especially the erythematous variant.
Alsaeedi and coworkers reviewed nine randomized clinical trials with a total of 3976 patients with chronic obstructive pulmonary disease treated with inhaled corticosteroids. They reported a risk ratio of 2.1 for oral Candida infections in patients treated with inhaled corticosteroids. It is postulated that steroids suppress localized cellular immunity and phagocytosis, thereby promoting the establishment of Candida. Likewise, impaired salivary flow may also predispose an individual to candidiasis through a reduced presence of salivary antimicrobial proteins such as lactoferrin, sialoperoxidase, and lysozyme. Moreover, salivary flow serves to dilute and remove potential pathogenic organisms from the oral mucosa. Thus, decreased salivary flow may be associated with oral candidiasis. Xerostomia, a subjective complaint of oral dryness, is a reported side effect of more than 500 medications. Xerostomia does not always correlate with a decrease in salivary gland output. There are medications known to decrease salivary output, and most have anticholinergic properties. The number of medications known to decrease salivary output is vast and includes antihistamines, tricyclic antidepressants, certain antihypertensives, hypnotics, and sedatives.
Similarly, systemic medications can affect the local environment in the oral cavity. Broad-spectrum antibiotics increase susceptibility to Candida infection by altering local oral flora that naturally inhibit candidal growth. Other systemic medications associated with onset of oral Candida infections include antineoplastic agents, immunosuppressive agents (such as azathioprine and glucocorticosteroids), and anticholinergic agents. Other systemic factors that have been implicated in oral candidiasis include smoking, diabetes mellitus, endocrinopathies, immunosuppressive conditions, malignancies, and nutritional disorders.
Diagnosis of oral Candida infections
Superficial Candida infections usually have a characteristic clinical appearance, and diagnosis is often based on clinical findings. Subjective complaints of localized oral burning are sometimes empirically treated for candidiasis.
Objective assays in the diagnosis of oral candidiasis include exfoliative cytology, imprint culture, swab culture, salivary assays, and mucosal biopsy. Exfoliative cytology is useful in differentiating between yeast and hyphal forms of Candida but is less sensitive than culture methods. A representative sample from the infected site is transferred to a microscopic slide and is stained with either potassium hydroxide (KOH), Gram’s stain, or periodic acid–Schiff (PAS) stain. Candidal hyphae appear as clear tubes with KOH staining because the KOH clears organic material from the background, making the fungi more readily apparent. With Gram’s staining, hyphae and yeast appear dark blue; PAS-stained specimens appear red to purple.
Imprint culture uses a sterile foam pad treated with growth media. The pad is placed on the infected site and then transferred to an agar medium for culture. Similarly, contents from an oral swab of the suspected site are transferred to growth media and cultured for candidal growth. Salivary rinse techniques involve the patient’s rinsing with a sterile phosphate-buffered saline rinse. The expectorant is concentrated by centrifugation, and the sample is inoculated on an agar growth plate.
Mucosal biopsy also can be used to ascertain Candida infection. An incisional biopsy of the suspected site should be placed in 10% formalin and submitted to an oral pathologist. The clinician should communicate with the pathologist the suspected fungal condition so special staining techniques such as PAS can be ordered. Typically the pathologist notes presence of yeast and hyphae in the keratin layer of the candidal biopsy specimen.
General characteristics of fungi seen on direct microscopic specimen examination include budding yeast cells (eg, blastoconidia), pseudohyphae, and hyphal structures. Budding yeast cells and pseudohyphae are characteristic of most Candida species and occur in a few other genera, such as Trichosporum and Geotrichum. Budding yeast cells without pseudohyphae are characteristic of C glabrata, Cryptococcus species, and Histoplasma capsulatum.
Exfoliative cytology, swab cultures, and incisional biopsy can be used to determine the presence of oral Candida species qualitatively, but these techniques are less sensitive than culture methods. Imprint culture and salivary rinse techniques allow quantification of Candida and can help differentiate between normal oral candidal levels and increased levels
associated with oral candidal infections. Regardless of the technique chosen, a microbiology or oral pathology laboratory service is required for a definitive diagnosis. Because of the increased costs associated with these assays and difficulty assessing required laboratory services, many clinicians routinely treat oral candidal infections empirically.
DEEP FUNGAL ORAL INFECTIONS
Other oral and perioral fungal infections are less commonly noted and are considered to be deep-seated fungal infections. In humans these infections include aspergillosis, cryptococcosis, histoplasmosis, geotrichosis, blastomycosis, and mucormycosis. These infections primarily affect sites other than the oral cavity and may be an indicator of systemic disease. Typically these deep-seated fungal infections are most commonly noted in immunosuppressed individuals such as those with HIV, AIDS, or malignancies. Diagnosis of these entities is confirmed through biopsy.
Aspergillosis
After Candida, aspergillosis is the second most common fungal infection found in humans. The incidence of invasive aspergillosis has increased dramatically in recent years secondary to an increase in the population of immunosuppressed individuals. Specific reasons include increased use of high-dose corticosteroids, worldwide increase in solid-organ and bone marrow transplantation, and increased use of immunosuppressive regimens for autoimmune diseases to ameliorate the host response system. The Aspergillus spore is found in decaying vegetation and in the immunocompromised host may cause acute invasive pulmonary aspergillosis. Aspergillus species have also been isolated from the air and environmental surfaces of hospital settings, particularly in areas undergoing structural renovation. Therefore hospitalized immunosuppressed individuals may be at increased risk for acquiring the disease. Aspergillus organisms tend to invade blood vessels causing thrombosis and infarction of the perivascular tissues. Less commonly Aspergillus invades the sinuses and can progress through the underlying soft tissue and bone to cause palatal and oral lesions, typically described as black or yellow necrotic lesions of the soft tissue.
Cryptococcosis
Cryptococcosis also primarily affects the lungs of immunosuppressed individuals. The causative agent, Cryptococcus neoformans, is a yeast that is found in bird droppings. The face, neck, and scalp are common sites of cutaneous lesions. Oral lesions are extremely rare but do occur. When present, the oral lesions may be superficial ulcerations, nodules, or granulomas or may have an ulcerated, indurated appearance resembling carcinoma.
Histoplasmosis
Histoplasma capsulatum is a dimorphic fungus with a yeast and mold form. Infection with H capsulatum can cause an acute or chronic respiratory illness or may present as a chronic mucocutaneous form. This mucocutaneous form may produce ulcerative, erosive lesions, which are more commonly noted on the tongue, palate, and buccal mucosa. According to Samaranayake, up to 50% of patients with the disseminated form of histoplasmosis have oral lesions, which frequently are the presenting sign of infection.
Geotrichosis
Geotrichum species are common inhabitants of the upper respiratory and gastrointestinal tracts and are found in up to 30% of healthy individuals. G candidum is responsible for geotrichosis, which may have oral, bronchial, intestinal, or pulmonary manifestations. Geotrichosis is a disease of immunocompromised individuals. Mucosal geotrichosis infections have pseudomembranous appearance and produce a chronic inflammatory reaction of the mucosa by surface pseudohyphae. Therefore geotrichosis should be included in the differential diagnosis of ulcerative inflammatory mucosal lesions of immunocompromised individuals.
Blastomycosis
Blastomycosis is one of the endemic mycoses in the central United States, caused by a dimorphic fungus, Blastomyces dermatitidis. The spore form of the fungus is found in soil, and if inhaled it may produce disseminated disease or a localized respiratory condition. The organism may produce epidemics following a point source of infection or sporadic endemic infection, but, generally, infection is a rare event. Blastomycosis is more commonly found in the Mississippi, Missouri and Ohio River valleys. When the disease is disseminated and affects the oral cavity, it produces ulcerating mucosal lesions.
Mucormycosis
Mucormycosis is an acute opportunistic infection caused by a saprophytic fungus found in soil, bread mold, decaying vegetation, and animal manure. Mucoraceae can be cultured from the oral cavity, nasal passages, throat, and stools of healthy individuals. Involvement of the oral cavity is usually secondary to infection of the paranasal sinuses or nasal cavity, usually presents as palatal necrosis or ulceration, and is noted in immunocompromised individuals. Early diagnosis and improved treatment has led to decreased morbidity and mortality. The
disease typically extends into adjacent structures causing extensive tissue necrosis and may extend into the brain. More recently Jones and coworkers report that mucormycosis can occur in patients with well-controlled diabetes mellitus with no other underlying immunosuppressive risk factors. These patients apparently have a higher rate of survival than other immunosuppressed patients.
Diagnosis of deep-seated oral fungal infections
Biopsy is routinely used to determine oral deep-seated fungal disease. A representative sample of tissue should be removed, placed in 10% formalin, and submitted to an oral pathology laboratory. The pathologist should be given appropriate information regarding the patient’s medical status, such as a known immunosuppressive state or medically induced immunosuppressive state. Additionally, the clinician should alert the pathologist of the suspicion of a deep-seated fungal infection so appropriate special staining may be ordered.
Deep-seated fungal diseases are usually signs of systemic disseminated disease, and thus patients with deep oral fungal infections must be referred to appropriate medical specialists for a further evaluation to determine underlying immune competency and central nervous system or pulmonary involvement. The development of more sensitive assays for fungemia detection is a major advance in the rapid diagnosis of invasive mycoses. Invasive fungal disease, particularly invasive yeast infection, is often first detected by a positive blood culture. Detection of fungal DNA and antigens seems to be the most promising technology for the detection of invasive fungal disease.
Most of the nonculture methods are currently investigational; these methods include polymerase chain reaction (PCR), galactomannan antigenemia, Western blot to detect antibodies, and detection of fungal metabolites (eg, Darabinitol and 1,3-b-D-glucan).
Treatment of oral fungal disease
Pharmacologic treatment for oral fungal diseases is primarily limited to three classes of antifungal agents: azoles, polyenes, and echinocandins. Another class of antifungal agents, pyrimidines, is sometimes used in concomitantly with azole or polyene agents. Before recommending or prescribing any medications, the clinician should review all medical information including use of prescriptive and over-the-counter medications and nutritional supplements. The following pharmacotherapeutic information is a general guide of medications available in the United States to treat fungal disease.
Azoles
Azole agents are divided into two groupings, imidazoles and triazoles. Azoles inhibit ergosterol, a main constituent of the fungal cell membrane, by inhibiting cytochrome P-450 (CYP)–dependent 14-a-demethylation. This inhibition results in fungal membrane changes that allow the cellular fungal elements to escape. Azole antifungal agents can cause CYP inhibition in humans. The CYP mixed-function oxidase enzymes are vital catalysts involved in metabolic breakdown and biotransformation of many drugs. As a result, azole agents can react with many other medications taken concomitantly.
Imidazoles
Within the imidazole group are clotrimazole, miconazole, and ketoconazole. Clotrimazole is available in various forms including an oral troche and topical rinse. There are also cream, lotion, and vaginal formulations. Clotrimazole alters cell membrane permeability by binding with phospholipids in the fungal cell membrane, allowing cellular elements to leak out. Clotrimazole is used to treat the erythematous and pseudomembranous variants of oral candidiasis. A cream formulation may also be used in the treatment of angular cheilitis. The oral troche contains 10 mg of clotrimazole. The patient should be instructed to let the troche dissolve in the mouth, which usually takes 15 to 30 minutes. Subsequent concentrations of clotrimazole sufficient to inhibit most species of Candida are present in saliva for up to 3 hours after dissolution. For treatment of oral candidiasis, patients are advised to use clotrimazole five times daily for a 14-day period. Clotrimazole may also be used prophylactically to guard against oral candidiasis in patients who are immunocompromised as the result of medical treatment. For prophylaxis against oral Candida infection, patients are advised to let the 10-mg troche dissolve in the mouth three times daily. Dental prostheses should be removed before employing clotrimazole therapy. Acrylic-containing prostheses should be regularly disinfected, especially in individuals with a present Candida infection or individuals who are using antifungal agents prophylactically.
Miconazole is similar to clotrimazole in its biologic spectrum and is available in a topical and parenteral form. The parenteral form is indicated for the treatment of systemic candidiasis and cryptococcosis. Ketoconazole is also an imidazole antifungal agent and is used to treat oropharyngeal candidiasis, cryptococcosis, histoplasmosis, blastomycosis, and chronic mucocutaneous candidiasis. Ketoconazole was the first oral antifungal agent introduced as an alternative to parenteral amphotericin B and alters cell membrane permeability. It is usually fungistatic but at higher concentrations may be fungicidal. Bioavailability is dependent on gastric pH; that is, increased gastric pH is associated with a decreased drug bioavailability. Therefore medications that increase gastric pH affect ketoconazole bioavailability. Because oral candidiasis can usually be treated with topical therapy, ketoconazole is reserved for systemic infections or Candida infections that do not respond to initial topical therapy.
Triazoles
Triazoles also are azole agents and have some advantages over imidazole agents. They have increased solubility and specificity for fungal enzyme systems, making them less toxic to the mammalian host. Fluconazole is effective in treating a variety of mycoses in both immunocompromised and immunocompetent hosts. Moreover, fluconazole is well tolerated and is not dependent on gastric pH. Fluconazole is used in the treatment of mucosal candidiasis, cryptococcal meningitis, and histoplasmosis. It is available in parental, oral, tablet, and oral suspension forms. Itraconazole, another member of the triazole family, has less toxicity and a broader spectrum of activity. It requires a low gastric pH for bioavailability and is well tolerated. The oral capsule and parenteral forms are used in the treatment of systemic fungal infections, including blastomycosis, histoplasmosis, and aspergillosis. The oral capsule should be given with food. Itraconazole oral solution is used for the treatment of oropharyngeal and esophageal candidiasis.
Voriconazole is the newest triazole agent available and is indicated for the treatment of aspergillosis. Voriconazole is a synthetic derivative of fluconazole and has expanded activity compared with fluconazole. It is also active against all Candida species, including C krusei, C glabrata, and fluconazole-resistant strains of C albicans. Voriconazole is available in a parenteral and oral tablet form.
Polyenes
Polyene antifungal agents include amphotericin B and its various formulations and nystatin. Nystatin is used in the treatment of localized oral fungal infections caused by Candida species, and amphotericin B and formulations are used in the treatment of severe deep-seated fungal disease. The polyene antifungals bind to ergosterol, a main constituent of fungal cell membranes, which results in loss of selective membrane permeability. The contents of the fungal cell leak out; the result is fungal cell death.
Amphotericin B
Amphotericin B is produced by Streptomyces nodosus and has a broad spectrum of activity including almost all yeasts and filamentous fungi. Side effects, mainly related to the infusion and nephrotoxicity, occur in 50% to 90% of patients. Because of these adverse effects many clinicians are reluctant to use the drug. At one time topical forms of amphotericin B (creams and mouthrinses) were commercially available in the United States.
Currently, no topical forms are available in the United States. More recent formulations of amphotericin B include the lipid complexes (ABLC), cholesteryl sulfate (ABCD), and liposomal forms (L-AmB). These newer formulations are less toxic, and some can be administered more rapidly and at higher doses than the conventional amphotericin B.
Nystatin
Nystatin is probably the most widely used agent to control topical Candida infection. It is available in creams, gels, oral rinses, and pastilles. The rinse and pastille forms contain sucrose and may promote caries in susceptible individuals. A sucrose-free vaginal tablet is available, as is an oral tablet, however patient compliance with this treatment is generally low because of the bitter taste. The troche formulation has a longer duration of action than the vaginal tablet, mainly because of the time required for the troche to dissolve. The oral rinse is relatively ineffective in treatment of oral candidiasis because of the short contact time with the mucosa.
Echinocandins
The Food and Drug Administration approved the drug caspofungin in January 2001. Caspofungin is categorized as an echinocandin, a separate class of antifungals. Unlike mammalian cells, fungal cell walls are composed of b-(1,3)-D glucan, mannan, and chitin. Echinocandins act to inhibit the b-(1,3)-D glucan synthase enzyme complex, thereby interfering with fungal cell wall synthesis. Caspofungin is indicated in the treatment of invasive aspergillosis in patients who do not respond to or are unable to tolerate other therapies such as amphotericin B and formulations of itraconazole. Caspofungin also has been shown to have fungicidal activity against Candida and Aspergillus species. Caspofungin is limited presently to a parental formulation.
Pyrimidines
Flucytosine is an antimetabolite drug that inhibits fungal protein synthesis by replacing uracil in fungal RNA with 5-fluorouracil. Flucytosine has activity against some strains of Candida, Cryptococcus, and Aspergillus. In the United States, flucytosine is administered orally and usually in conjunction with another antifungal agent such as amphotericin B, fluconazole, or itraconazole for the treatment of severe candidal or cryptococcal infections.
SUMMARY
Candidiasis is the most common oral fungal infection diagnosed in humans. Candidiasis may result from immune system dysfunction or as a result of local or systemic medical treatment. Because oral candidiasis is generally a localized infection, topical treatment methods are the first line of therapy, especially for the pseudomembranous and erythematous variants. Patients with dental prostheses should also be advised to disinfect the prosthesis routinely during the candidal treatment period, because the prosthesis may serve as a source of reinfection. Additionally, patients should be advised that oral hygiene aids, such as toothbrushes and denture brushes, may also be contaminated and should be discarded or disinfected. A disinfecting solution of equal parts of hydrogen peroxide and water may be used. Likewise, 2% chlorhexidine gluconate solution may be used as a disinfecting solution for dental prostheses and oral hygiene aids.
Occasionally the clinician encounters a more resistant form of oral candidiasis such as the hyperplastic variant or a variant that does not respond to topical therapy. Appropriate systemic therapy should be employed for the treatment of these infections. Additionally, a biopsy should be undertaken in individuals with the hyperplastic variant of Candida because there is some degree of risk for malignant transformation.






Excellent article! We are linking to this particularly great content on our website. Keep up the great writing. Crystal Borden Brittney